Rugged Arm Tablet May Revolutionize Remote Medical Record Access

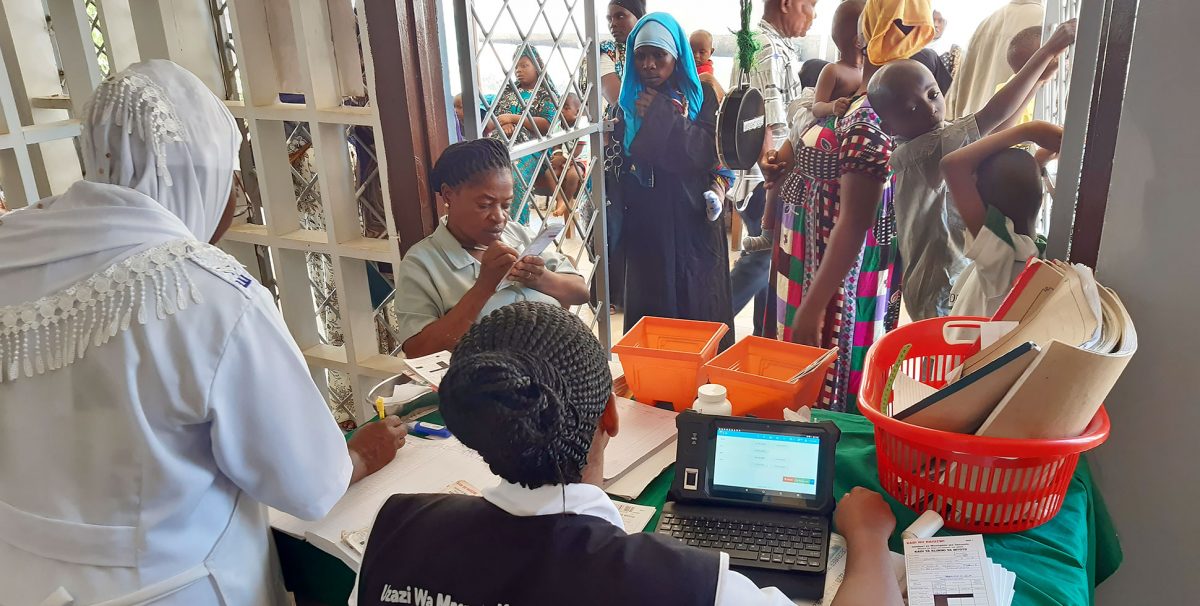
Neema is a community health worker in Tanzania. Responsible for the health and wellbeing of several remote villages, she carries a heavy ledger weighing tens of kilograms in a bag slung across her shoulder. The ledger contains the precious medical records of her charges, and she must take immense care with it: the entire medical history of her patients depends upon it.
Be He@lthy, Be Mobile (BHBM) is a joint initiative between the World Health Organisation (WHO) and the International Telecommunication Union (ITU). It’s working hard to deliver digital healthcare to communities like Neema’s – both to reduce the burden on workers, and to provide a permanent, centralised record of the provision of care. But while workers in city facilities can swap heavy paper ledgers for lightweight tablets, those on the road face a challenging working environment that creates numerous obstacles to tablet adoption.
Many remote locations have an unreliable electrical supply, if one exists at all; the ability to charge a device isn’t a given. In Tanzania, for example, 73 percent of the population lives in rural areas and only 32 percent of households have access to electricity.
Working environments present additional physical challenges: high ambient temperatures trigger speed throttling as devices slow themselves down in an effort to prevent overheating. Heat also significantly reduces battery life and can even cause damage to the device’s casing. In extremely dry conditions, dust can cause problems, too, causing devices to overheat or blocking charge ports. And that’s not to mention the most fragile part of any tablet – its screen.
Finding a commercial device at an affordable price that could withstand these conditions proved to be a challenge, so BHBM decided to ask Arm for help.
Scoping the Requirements
Arm is a long term supporter of BHBM, a global initiative targeting disease prevention through mobile technology. This challenge gave us an opportunity to strengthen our partnership, as well as a new way to ensure Arm technology helps realise the United Nations’ Global Goals.
Despite the direct design and manufacture of an endpoint device being outside Arm’s usual remit as an IP design company, we knew immediately that we wanted to be involved: if we could design a device that met the requirements, it could quite literally revolutionise the lives of these last-mile health workers and the level of care they could offer to their patients. But we wanted to be sure that we were investing in a device that was fit for purpose, so we carried out 96 interviews with experts and healthcare workers across Bangladesh, Tanzania and Zambia to understand exactly what their needs were.
This gave us our ideal specification: a rugged Arm tablet that has a battery life of three to four days, a front and back camera with flash for simple diagnostics and rugged casing to withstand heat, water and dust.
We then mapped this specification to the closest 45 off-the-shelf rugged tablet devices, but none met more than sixty per cent of our requirements. Further research led us to high-end industrial-grade devices which, while closer to what we wanted, were expensive and over-specified for our needs.
It was at that point we decided that if the device didn’t exist, then perhaps we should help invent it. And thanks to the support of the Department for International Development’s (DfID) Business Partnership Fund we were able to do just that.
Defining the specification
We drew on the field knowledge of BHBM, the development and management experience of Accenture Development Partnerships, pro bono legal advice from international law firm Bird & Bird and the technical expertise of Arm engineering, research, legal, trade compliance and development solutions teams.
Having input from such a wide range of people with a broad spectrum of specialist skills played a significant role in shaping the prototype device. People who do this regularly know that a device must achieve a certain level of compliance and certification before it’s considered safe to use. That’s where having in-house advice on risk assessments and compliance came into its own.
Making it real
Finding a manufacturer willing to develop a tablet prototype at a price-point that development agencies and governments could afford also proved to be quite a challenge. But once we got over that hurdle, it was tremendously exciting to know that we were one step closer to making this solution a reality.
We’re currently analysing the results from the prototype’s field tests with community healthcare workers in Bangladesh and Tanzania, facilitated by WHO partners mPower and PATH. The initial results are very promising, with the most positive feedback coming from the device’s significantly increased battery life. With Arm-based mobile devices already offering multi-day battery life to consumers, it was only a matter of refining the Arm technology within the rugged Arm tablet to maximize this. Workers using the prototype repeatedly commented about the improved healthcare service and the increased number of patients they think the device could help them provide.
Our research into market demand has also shown that while we designed this rugged Arm tablet for the healthcare sector primarily, it has the potential to be a useful tool in agriculture, education, field conservation and disaster relief. We’re now working to identify the right set of partners to take the project forward. Watch this space.
Any re-use permitted for informational and non-commercial or personal use only.














