TTP CoTest: Arm-powered Medical Cloud Enables Rapid Rollout of COVID-19 ‘Lab-in-Box’

It takes three to seven years on average to bring a new medical device to market, costing on average $39m. In the world of diagnostic systems, there are many cases whether both the cost and timescales considerably exceeded these numbers.
It is a challenging process, not only from an engineering, biology, and software development standpoint but in proving the security and safety that the regulations mandate. These are absolutely necessary to ensure that medical devices can be trusted to perform reliably and accurately in their day-to-day operation and won’t leak or corrupt sensitive patient data.
In the case of modern connected medical systems, it is not only the device itself that causes regulatory challenges: it’s the way it interfaces with medical records and other sensitive systems within a healthcare setting.
A new prescription for medical technology
At The Technology Partnership (TTP), our team of 250+ scientists and engineers specialize in the rapid design and deployment of devices and solutions for clients across a range of market sectors – from satellite communications to drug discovery tools. In diagnostics, drug delivery, surgical tools and other regulated medical markets we develop the devices and solutions to deliver suitable and medically valuable performance. But developing in a regulated environment always adds to the overall programme timeline.
So when we started to develop CoTestTM, a ‘lab in a box’ designed for rapid screening of up to 50 COVID-19 samples at once, we were well aware that it would be difficult to obtain regulatory approval in time to make a real difference in the fight against the virus.
We envisaged TTP CoTest to be as simple as possible to use by end-users, be they staff and students in a school classroom or patients and clinicians in a resource-limited setting
Our search for a solution led us to L2S2 and their medical device cloud (MMDC), a cloud data platform that aggregates medical data from a range of digital health devices without the need for those devices to have been individually certified or accredited by each medical facility.
All it took was implementing L2S2’s communications hardware into the prototype TTP CoTest system. From receiving the hardware on a Friday afternoon, we had a CoTest prototype connected to the MMDC and ready to deliver secure, medical-grade data to healthcare providers by Monday lunchtime.
TTP CoTest: A COVID-19 test lab in a box
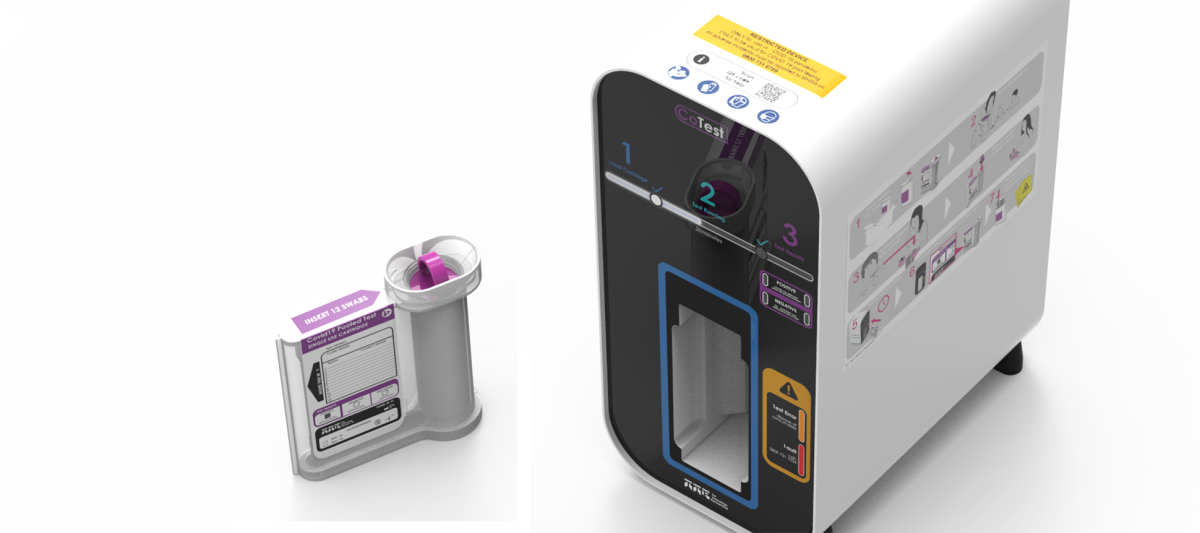
We envisaged TTP CoTest to be as simple as possible to use by end-users, be they staff and students in a school classroom or patients and clinicians in a resource-limited setting. Saliva samples from up to 50 people is pooled within a disposable cartridge using our bespoke sample preparation technology, a far less intimidating and costly process than a nasal swab and one that people can perform on their own.
The cartridge is then inserted into TTP CoTest, an Arm-based system that resembles a mini-tower desktop computer. The TTP CoTest box then performs rapid RNA amplification testing through a series of liquid handling, isolation, heating and detection procedures, carefully calibrated by software within the device. It’s far faster than sending off for a PCR result yet more sensitive and cheaper in continued use than lateral flow testing.
A positive test will announce that at least one person in the pool is positive – and actions can be taken to isolate the whole cohort and then testing of smaller pools and individuals can allow for rapid and safer identification of asymptomatic positives, thereby curbing transmissions, while people who haven’t been infected can continue to lead normal lives.
The CoTest device quickly demonstrated its viability. By tethering it locally to a desktop PC we could demonstrate the accuracy and reliability of the system we were developing. But enabling that data to be transferred to a wider network so that a healthcare provider could make clinical sense of it meant bringing it online within a healthcare setting, and we knew that we were already on the regulatory back foot.
Managed Medical Device Cloud: The middle-man
That’s when we turned to CEO Philip Gaffney and his team at L2S2. In January 2021, Arm’s Peter Ferguson explained on Arm Blueprint how stringent standards, alongside proprietary technology, are impeding innovation in the healthcare industry. Few medical devices in use today have been built with a platform approach in mind, says Ferguson. They’re built from the ground up using proprietary technology, with software instructions executed directly on ‘bare metal’ hardware. They communicate via any combination of Bluetooth, Wi-Fi and cellular to deliver data using their own security standards with almost no standardization.
L2S2’s MMDC medical data and analytics SaaS platform had been developed to bring all of this chaos to order through a platform approach that helps streamline the compliance process for new medical devices and services, eliminating the need for engineers to start from a blank slate.
This wasn’t a case of a company greasing the wheels or promising accelerated approvals processes by pulling strings. With MMDC, L2S2 could provide accredited turnkey compliance via an instant, secure and trusted connection to medical systems in order to deliver data to healthcare professionals.
Even before the CoTest prototype was fully functional, L2S2 and TTP had generated simulations of the type of data we expected to collect and built an interface for us. L2S2 was also able to provide remote controls for the CoTest device – telling the device’s servo motors and actuators exactly how to move and how fast and how rapidly to track temperature for isothermal testing of COVID-19 samples.
Alongside the MMDC cloud platform underpinned by Arm technology, L2S2 had developed a portfolio of Arm-based hardware development boards, pre-built and pre-approved by regulators that could be fitted inside existing medical devices, new or old.
We knew that being Arm, these devices already had everything they needed to interface and communicate with the hardware we’d built into CoTest. Suddenly, a development process that we were used to progressing in a serial fashion could be performed in parallel with everything else.
The development board acts as a gateway to MMDC’s systems, using a single wire to communicate data between the host device and the L2S2 board. Very simple instructions are all that is required to exchange medical data.
That’s where we found ourselves that Friday afternoon, having connected CoTest: the L2S2 team in Italy feeding data into the system and actuating the CoTest prototype in Cambridge then feeding the results back to Italy.
L2S2 MMDC: A game-changer for connected diagnostics
Adding approved, medical-grade connectivity to our project was a game-changer – not just for CoTest but as a major enabler for future developments for TTP’s clients in connected diagnostics and medical devices. Now, medical professionals can monitor patients on a weekly or even daily basis with very little training required from anywhere in the world.
MMDC also gained us a significant advantage during the scientific discovery phase of CoTest’s development. Scientists often use logbooks (physical or electronic) for developing and testing new sensors and chemistry. Changes are recorded and information from that process feeds the next test – but not all pieces of data are noted and not everything is timestamped. When the product development moves to a regulated environment some of that logbook information needs to be recreated and retested with more information captured.
With MMDC, we had every test script and test result automatically recorded from day one of development. This seemingly small benefit of connectivity resulted in the saving of thousands of pages of regulatory submission with the system being able to automatically generate reports and test results.
TTP CoTest is a diagnosis platform for tomorrow
How many medical devices are at risk of ending up the ‘cupboard of doom’ because they are no longer compatible with medical IT systems and protocols or modern healthcare processes?
TTP CoTest may have been developed for current strains of COVID-19, but it was always developed with the future in mind.
While CoTest makes use of isothermal testing for viral RNA in COVID-19, we’re not limited to that method. Our cartridge system means we can adapt CoTest to take samples of blood or media collected from swabs.
Should more accurate detection methods be discovered, new variants appear or other diseases need identification through bacterial RNA, DNA or even protein analysis, we can swap out the cartridge to immediately update the system.
Any required protocol changes can be delivered automatically via the MMDC cloud through over-the-air (OTA) firmware updates. In this way, CoTest can be updated and tweaked to perform detection of diseases now and many years into the future.
Beyond CoTest, MMDC opens up a world of innovation for my team and any other company currently stumbling over the many regulatory hurdles required to get much-needed networked medical devices into the hands of healthcare professionals. Unbridled by years of approvals, we expect we will be able to develop and deploy medical devices of huge value to professional healthcare far more quickly than we could ever have dreamed of before.
As Phillip Gaffney said to me last time we spoke, this isn’t the end of a journey it’s the beginning. The collaboration, enabled by Arm, has been astounding. It’s built confidence that we have an approach which may well radically change the way diagnostic devices are developed in the future and ensure that we’re able to meet urgent demands created by whatever comes next.
Healthcare Technology for Improved Insights and Outcomes
Innovations in healthcare technology can help transform healthcare from reactive to proactive. Arm provides cost-effective, foundational technology for secure, low-power, high-performance electronic devices across healthcare to help improve patient outcomes and people’s lives.
Any re-use permitted for informational and non-commercial or personal use only.












